This is a prospective, multicenter, double-blind, randomized, sham-controlled study to demonstrate the superiority of treatment with the Nuvaira Lung Denervation System compared to a sham procedure to decrease moderate or severe exacerbations in subjects with COPD on optimal medical care.
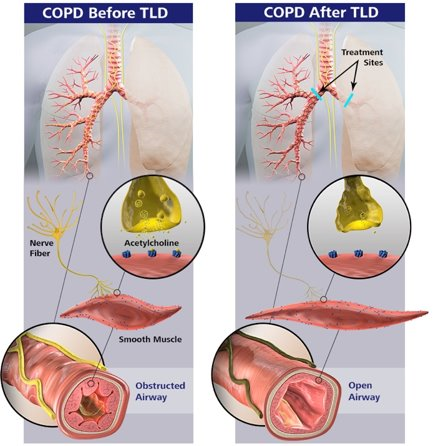
The procedure involves bronchoscopic ablation of pulmonary vagus nerve surrounding the main bronchi. This inhibits vagal reflexes responsible for smooth muscle constriction and mucus secretion.
For more detailed information on this trial please click the link above to visit ClinicalTrials.gov