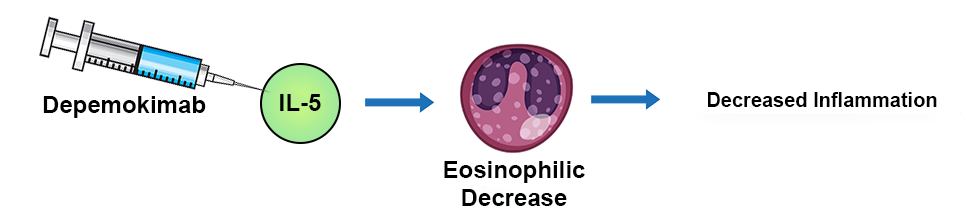
This is a Phase III, randomized, placebo controlled study evaluating the efficacy and safety of depemokimab in patients with COPD GOLD E and elevated eosinophils.
Depemokimab is monoclonal antibody selectively binding to interleukin-5 which is a central driver of eosinophilic inflammation in airway diseases. It is an ultra-long-acting biologic, allowing for dosing as infrequently as twice yearly by providing sustained inhibition of IL-5 and continuous reduction of eosinophil counts.
For more detailed information on this trial please click the link above to visit ClinicalTrials.gov